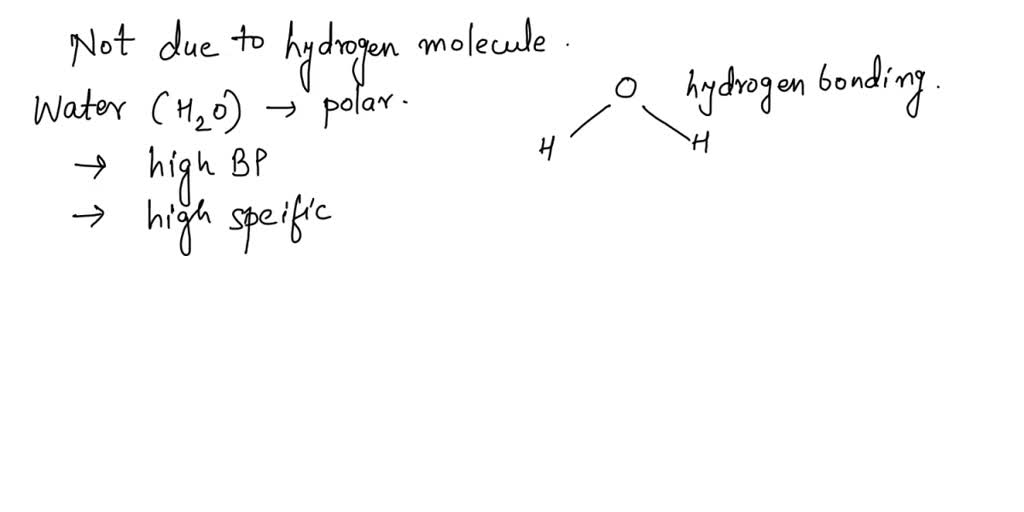
Water Molecule Surface Tension Property . Besides mercury, water has the highest surface tension for all liquids. In the current ocean, ph is about 8.1, which leads to about. revision notes on 1.6.3 the properties of water for the aqa a level biology syllabus, written by the biology experts at save my exams. surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water. It’s even possible to “float” a needle on top of a. cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. Water has some unusual properties due to the hydrogen bonding between its molecules. surface tension, heat of vaporization, and vapor pressure. The density of ice is less than water. since these intermolecular forces vary depending on the nature of the liquid (e.g. Gasoline) or solutes in the liquid (e.g. the equilibrium between the species depends on the ph. the properties of water.
from www.numerade.com
surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water. the equilibrium between the species depends on the ph. revision notes on 1.6.3 the properties of water for the aqa a level biology syllabus, written by the biology experts at save my exams. Gasoline) or solutes in the liquid (e.g. In the current ocean, ph is about 8.1, which leads to about. The density of ice is less than water. Besides mercury, water has the highest surface tension for all liquids. Water has some unusual properties due to the hydrogen bonding between its molecules. since these intermolecular forces vary depending on the nature of the liquid (e.g. It’s even possible to “float” a needle on top of a.
SOLVED Which of the following properties is not due to the hydrogen
Water Molecule Surface Tension Property Water has some unusual properties due to the hydrogen bonding between its molecules. the properties of water. It’s even possible to “float” a needle on top of a. since these intermolecular forces vary depending on the nature of the liquid (e.g. surface tension, heat of vaporization, and vapor pressure. Water has some unusual properties due to the hydrogen bonding between its molecules. surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water. the equilibrium between the species depends on the ph. In the current ocean, ph is about 8.1, which leads to about. Gasoline) or solutes in the liquid (e.g. revision notes on 1.6.3 the properties of water for the aqa a level biology syllabus, written by the biology experts at save my exams. Besides mercury, water has the highest surface tension for all liquids. cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. The density of ice is less than water.
From www.dreamstime.com
Surface Tension Explanation Vector Illustration Diagram Stock Vector Water Molecule Surface Tension Property Water has some unusual properties due to the hydrogen bonding between its molecules. surface tension, heat of vaporization, and vapor pressure. It’s even possible to “float” a needle on top of a. the equilibrium between the species depends on the ph. In the current ocean, ph is about 8.1, which leads to about. Gasoline) or solutes in the. Water Molecule Surface Tension Property.
From factsandhistory.com
Why Does Water Feel Like Concrete When You Belly Flop Into It Water Molecule Surface Tension Property It’s even possible to “float” a needle on top of a. cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. Water has some unusual properties due to the hydrogen bonding between its molecules. The density of ice is less than water. the equilibrium between the species depends. Water Molecule Surface Tension Property.
From exoziqnph.blob.core.windows.net
What Is The Surface Tension Of Water Called at Jonathan Blackburn blog Water Molecule Surface Tension Property the properties of water. surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water. Water has some unusual properties due to the hydrogen bonding between its molecules. revision notes on 1.6.3 the properties of water for the. Water Molecule Surface Tension Property.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Water Molecule Surface Tension Property It’s even possible to “float” a needle on top of a. In the current ocean, ph is about 8.1, which leads to about. the properties of water. Besides mercury, water has the highest surface tension for all liquids. revision notes on 1.6.3 the properties of water for the aqa a level biology syllabus, written by the biology experts. Water Molecule Surface Tension Property.
From www.slideserve.com
PPT The Properties of Water PowerPoint Presentation, free download Water Molecule Surface Tension Property Water has some unusual properties due to the hydrogen bonding between its molecules. Gasoline) or solutes in the liquid (e.g. surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water. In the current ocean, ph is about 8.1, which. Water Molecule Surface Tension Property.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it Water Molecule Surface Tension Property Water has some unusual properties due to the hydrogen bonding between its molecules. surface tension, heat of vaporization, and vapor pressure. In the current ocean, ph is about 8.1, which leads to about. the properties of water. The density of ice is less than water. since these intermolecular forces vary depending on the nature of the liquid. Water Molecule Surface Tension Property.
From pdfslide.net
(PPT) Structure & Properties of Water. STRUCTURE OF WATER POLAR Water Molecule Surface Tension Property The density of ice is less than water. surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water. Water has some unusual properties due to the hydrogen bonding between its molecules. the equilibrium between the species depends on. Water Molecule Surface Tension Property.
From www.slideserve.com
PPT Understanding Water PowerPoint Presentation, free download ID Water Molecule Surface Tension Property Besides mercury, water has the highest surface tension for all liquids. The density of ice is less than water. surface tension, heat of vaporization, and vapor pressure. revision notes on 1.6.3 the properties of water for the aqa a level biology syllabus, written by the biology experts at save my exams. In the current ocean, ph is about. Water Molecule Surface Tension Property.
From www.slideshare.net
Measuring the Surface Tension of Water by Light Diffraction on Capill… Water Molecule Surface Tension Property the equilibrium between the species depends on the ph. The density of ice is less than water. since these intermolecular forces vary depending on the nature of the liquid (e.g. surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature. Water Molecule Surface Tension Property.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Water Molecule Surface Tension Property It’s even possible to “float” a needle on top of a. Water has some unusual properties due to the hydrogen bonding between its molecules. cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. the properties of water. Gasoline) or solutes in the liquid (e.g. surface tension. Water Molecule Surface Tension Property.
From www.slideserve.com
PPT Water Chemistry PowerPoint Presentation, free download ID5763752 Water Molecule Surface Tension Property Besides mercury, water has the highest surface tension for all liquids. The density of ice is less than water. cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. surface tension, heat of vaporization, and vapor pressure. In the current ocean, ph is about 8.1, which leads to. Water Molecule Surface Tension Property.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces Water Molecule Surface Tension Property the equilibrium between the species depends on the ph. Water has some unusual properties due to the hydrogen bonding between its molecules. since these intermolecular forces vary depending on the nature of the liquid (e.g. It’s even possible to “float” a needle on top of a. The density of ice is less than water. Gasoline) or solutes in. Water Molecule Surface Tension Property.
From slideplayer.com
Solutions Chapter 15 Chapter ppt download Water Molecule Surface Tension Property Besides mercury, water has the highest surface tension for all liquids. the equilibrium between the species depends on the ph. cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. since these intermolecular forces vary depending on the nature of the liquid (e.g. Gasoline) or solutes in. Water Molecule Surface Tension Property.
From ar.inspiredpencil.com
Surface Tension Of Water Molecules Water Molecule Surface Tension Property the equilibrium between the species depends on the ph. Water has some unusual properties due to the hydrogen bonding between its molecules. cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. In the current ocean, ph is about 8.1, which leads to about. surface tension, heat. Water Molecule Surface Tension Property.
From botnam.com
Structure Of Water Molecule Properties Of Water (2024) Water Molecule Surface Tension Property the equilibrium between the species depends on the ph. Gasoline) or solutes in the liquid (e.g. It’s even possible to “float” a needle on top of a. In the current ocean, ph is about 8.1, which leads to about. since these intermolecular forces vary depending on the nature of the liquid (e.g. revision notes on 1.6.3 the. Water Molecule Surface Tension Property.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Water Molecule Surface Tension Property The density of ice is less than water. Gasoline) or solutes in the liquid (e.g. the equilibrium between the species depends on the ph. surface tension, heat of vaporization, and vapor pressure. revision notes on 1.6.3 the properties of water for the aqa a level biology syllabus, written by the biology experts at save my exams. . Water Molecule Surface Tension Property.
From www.numerade.com
SOLVED Which of the following properties is not due to the hydrogen Water Molecule Surface Tension Property Besides mercury, water has the highest surface tension for all liquids. surface tension could be defined as the property of the surface of a liquid that allows it to resist an external force, due to the cohesive nature of the water. Water has some unusual properties due to the hydrogen bonding between its molecules. since these intermolecular forces. Water Molecule Surface Tension Property.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii Water Molecule Surface Tension Property It’s even possible to “float” a needle on top of a. cohesion and surface tension keep the hydrogen bonds of water molecules intact and support the item floating on the top. the equilibrium between the species depends on the ph. Water has some unusual properties due to the hydrogen bonding between its molecules. Gasoline) or solutes in the. Water Molecule Surface Tension Property.